LAST Friday 5 November 2021, the drug giant, Pfizer, announced that it had developed an antiviral pill, which had undergone critical clinical trial and was found to reduce the risk of hospitalisation or death from COVID-19 by 89%! The drug if approved by the US Food and Drug Administration (FDA), would be the second pill after Merck’s Molnupiravir, to be deployed against COVID-19. The drug is to be marketed under the trade name, PAXLOVID™.
Previously another drug giant, Merck, announced on Friday 1 October 2021 that it had produced the first oral antiviral pill for treating COVID-19 infection. The drug called Molnupiravir, received approval on Thursday 4 November 2021 from the UK Medicines and Healthcare Products Regulatory Agency. Approval for the drug is still being awaited from the FDA in the US. Merck’s pill is approved for use in the UK for people at high risk of severe illness from COVID-19.
The Pfizer pill, PAXLOVID™, according to data presented by Pfizer, is so demonstrably effective against COVID-19 that an independent Data Monitoring Committee, in consultation with the U.S. FDA, recommended that further enrolment into the study be discontinued. Pfizer is currently planning to make all the data with respect to the new drug, available to the U.S. FDA for Emergency Use Authorisation (EUA).
Join our WhatsApp ChannelPAXLOVID™ is described by Pfizer as an oral SARS-CoV-2 protease inhibitor antiviral therapy. And should be prescribed at soon as infection is detected in order to assist patients avoid the severe illness seen in COVID-19 cases, which often leads to hospitalisation or death. The drug blocks an essential enzyme seen in Coronavirus. This enzyme is called 3CL protease, which the virus uses to process its polyproteins during replication. Therefore, by blocking this important enzyme during proteolysis, the virus is unable to replicate and cause infection. But the drug does require a helper drug to remain active at high concentrations in the body for longer periods of time. The helper drug used by Pfizer is an old drug used for the treatment of H.I.V infection called ritonavir. It slows the rate at which the body metabolises (or breaks down) the drug, PAXLOVID™, and by doing this ensures that PAXLOVID™ remains in higher concentrations in the body for a longer period, enough to enable the drug tackle the virus.
The tests were conducted between July and September at the height of the Coronavirus Delta variant pandemic. The tests were conducted among more than 1200 high-risk adult patients both from the US and overseas. The test subjects who all had COVID-19 infection, unvaccinated, and were either old, had obesity or diabetes (i.e. they were at the high risk spectrum of becoming severely ill from COVID-19 leading to hospitalisation or death), received either the Pfizer’s drug, PAXLOVID™, or a placebo (dummy) pill. The test subjects were treated with 30 pills over a 5-day period within 3 to 5 days after contracting COVID-19. Ritonavir was included in the treatment regime to enhance the activity of the drug. In total, a person needs to take 3 tablets twice a day at 12 hours intervals for 5 days. Less than 1% of those given the drug were hospitalised but no death was recorded. Whereas 7% of those given placebo were either hospitalised or dead.
Pfizer previously developed the drug for use against the SARS virus but started in 2020 to modify it against COVID-19 instead. The efficacy of the drug at reducing hospitalisation or death was 89% in contrast to Merck’s Molnupiravir at 50% and monoclonal antibody at 70%. However, the efficacy comparisons are deceptive as the test conditions were different. For example, the Pfizer PAXLOVID™ was co-administered with ritonavir, which ensures its high concentrations in the body for a longer period, thereby boosting its effectiveness against the virus.
Unlike Merck’s Molnupiravir, which works by mutating the virus’s genome so that the virus is unable to replicate, Pfizer’s PAXLOVID™ does not cause “undue” mutations. But both pills do have some side effects, which are no worse than the disease itself.
“Today’s news is a real game-changer in the global efforts to halt the devastation of this pandemic. These data suggest that our oral antiviral candidate, if approved or authorised by regulatory authorities, has the potential to save patients’ lives, reduce the severity of COVID-19 infections, and eliminate up to nine out of ten hospitalisation,” gushed Albert Bourla, Chairman and Chief Executive Officer of Pfizer. “Given the continued global impact of COVID-19, we have remained laser-focused on the science and fulfilling our responsibility to help healthcare systems and institutions around the world while ensuring equitable and broad access to people everywhere.”
However, it is not everybody who is happy about the pills. The Merck’s pill, Molnupiravir, works by inserting mutations in the viral genome making it impossible for the virus to replicate. The fear here is that the drug could cause the same thing to happen to a person’s genetic material, which might lead to cancer or birth defects. Such fears it must be stressed are so far unfounded. Another cause for concern is the fear that by causing mutations in the viral genome, a new variant more dangerous than the current variants might arise.
These concerns arose due to the high incidence of antibiotic resistance seen in bacteria, which primarily is caused by antibiotic abuse. Often, prescribed course of antibiotics and other drugs are not completed by patients causing resistant bacteria to survive and spread. If the same thing happens with any of these pills, drug-resistant virus variants could arise.
Fears that antiviral drugs would cause the virus to increase its transmissibility or virulence are unfounded, according to Daria Hazuda, the head of Infectious Disease Discovery at Merck. In patients who completed the 5-day course of the drug, there has been no detection of dangerous variants of the virus except only random harmless mutations. It should be noted that Coronavirus is naturally prone to generating variants without any selective pressure during its replication in people.
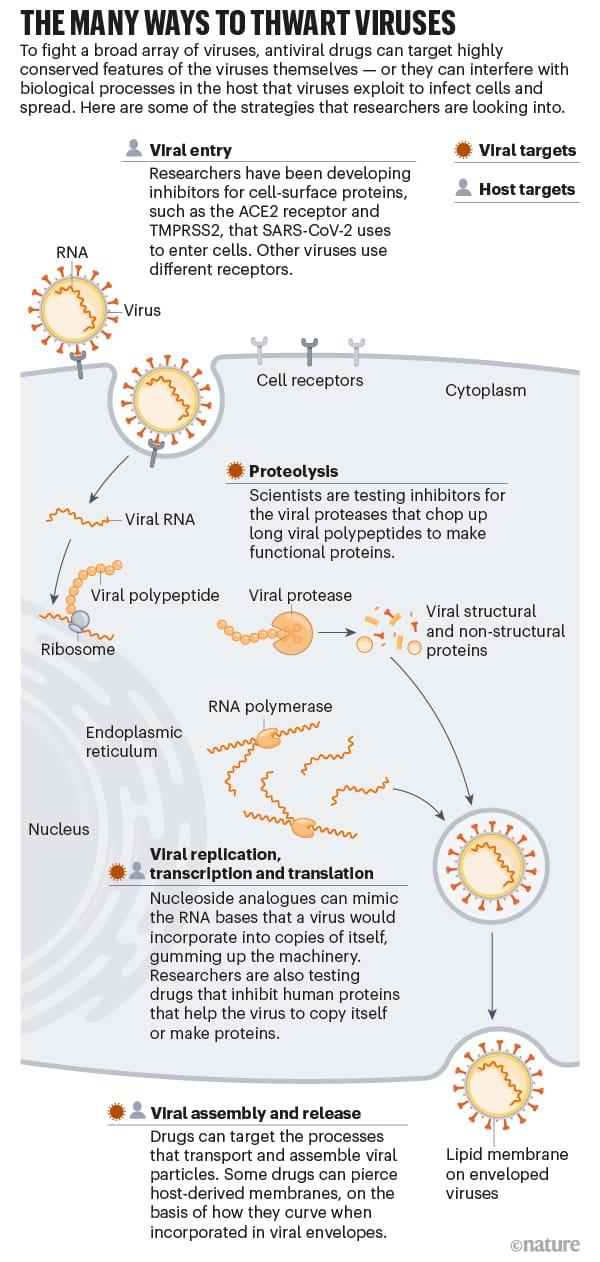
However, targeting viruses for drug design is not easy. Protozoa, fungi and bacteria are easily targeted by drug companies compared to viruses. This is because their cellular components and big genomes offer hits for drug activities. Contrast them with viruses, which have smaller genomes, no cellular structures, and replicate and mutate quite easily, it then becomes a nightmare to contain viruses easily with drugs as with bacteria, fungi and parasites.
Viral flexibility is a big concern because a drug developed for one type of virus does not always work against another. However, despite these innumerable obstacles, a lot of ways are being devised by scientists in tackling viruses (see figure produced by Nik Spencer in collaboration with Nature, above). And it is hoped that now that scientists are primed into action after the lessons of Coronavirus pandemic, future pandemics will easily be contained.
Pfizer’s new antiviral drug, PAXLOVID™, is expected to be available in the market by early next year subject to regulatory approval. The UK has already ordered 250,000 courses of the medication to help reduce the pressure on the NHS. Meanwhile about 46 million people in the UK have been fully vaccinated, and over 10 million have had their respective booster dose of Coronavirus (COVID-19) vaccine with yet another 3 million people invited for participation in the vaccine booster programme.
The question that comes to mind is, should we relax and celebrate given all that we have been through and the superhuman efforts we have made in containing the virus? We could all remember when dead bodies in body bags were being lined up in trailers in the US like firewoods because the mortuaries were full, and in India corpses were littered on the ground in open places waiting for cremations. All over the world the story was the same, people were dying of the virus in droves. The streets were empty, basic goods were scarce as people stayed indoors afraid to venture out for fear of catching the virus; social and economic lives ground to a halt. But thanks to the inherent ability of mankind to survive and master his environment, within a short while vaccines against the virus were discovered. Monoclonal antibodies were produced and now we have two different pills all designed to fight the virus. These efforts by mankind have helped return life to near normality.
Verdict: yes, we should pat ourselves a little on the back and wet our whistle a bit, but we shouldn’t get drunk and let our guards down. More pandemics might still be coming and we should use the lessons learnt with the Coronavirus pandemic to mitigate against future occurrences.




















Follow Us