THE Biblical Jesus, said to a lame man, “Rise, take up thy bed, and walk.” And immediately, according to the Bible, the man was made whole, and took up his bed, and walked. Ever since that time, man has been trying to recreate the same miracle but with no complete success. But a recent discovery might just be humanity’s answer to the Biblical miracle.
Each year as much as half a million people worldwide suffer from spinal cord injuries. Most of these injuries are from motor accidents or falls. Damage to the spinal cord or tissues and bones surrounding it can lead to paralysis depending upon the severity of the injury. And when this happens, the nerves that carry messages to and from the brain to the rest of the body are rendered useless leading to a complete loss of motor function. The person is said then, to be paralysed. But if the spinal cord is not completely damaged, it may still be able to carry out its function of transmitting messages, and in this case the person recovers after a while.
There are quite a number of studies into spinal cord injury repairs. Most of these studies focus on the use of electrical implants and stem cell injection for regeneration of damaged spinal cords. Currently, there are no known complete successful treatment of the condition, but there are therapies available to help manage the condition.
Join our WhatsApp Channel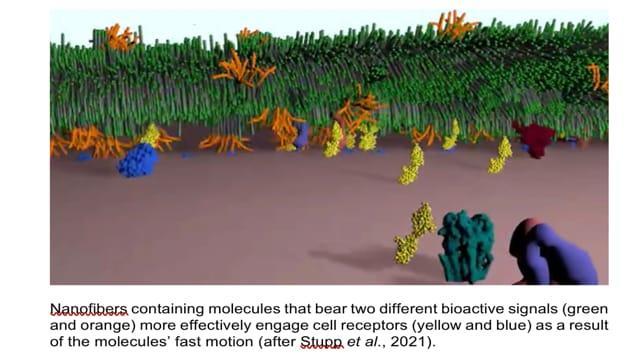 A paper published on Friday 12 November 2021, in the journal, Science, by Stupp and coworkers from the Northwestern University, USA, shows that it is possible for damaged spinal cord to regenerate. In the study, Stupp and co, coaxed tissue cells to repair and regenerate by injecting bioactive molecules into the site of the spinal cord injury.
A paper published on Friday 12 November 2021, in the journal, Science, by Stupp and coworkers from the Northwestern University, USA, shows that it is possible for damaged spinal cord to regenerate. In the study, Stupp and co, coaxed tissue cells to repair and regenerate by injecting bioactive molecules into the site of the spinal cord injury.
First, the researchers theorised that since cells and their receptors are perpetually moving about, the more rapid a molecule moves, the more chances of that molecule making contact with these receptors. Hence, they set about controlling the movements of these molecules to enable them seek and interact with the constantly mobile cellular receptors.
They built a network of nanofibres to mimic the extracellular matrix of the spinal cord. The nanofibres are made up of weakly bonded structural units of peptides that wiggle and vibrate. By keeping the bioactive molecules in perpetual motion, upon contact with cellular receptors that also are in perpetual motion, they were able to transmit signals to regenerate damaged spinal cords in mice.
The nanofibre scaffolds they created, bear two peptide sequences of proteins for tissue regeneration, one of which is responsible for reduction of glial (myelin) scarring, and the other influences the formation of new blood vessels.
The synthesised supramolecular peptide fibril scaffolds containing the bioactive molecules were introduced by injection in liquid form into the tissues surrounding the spinal cord. These form a gel that mimics the extracellular matrix of the spinal cord. By mimicking the natural environment of the spinal cord so perfectly, the bioactive molecules were able to communicate with cells and trigger them to repair and regenerate.
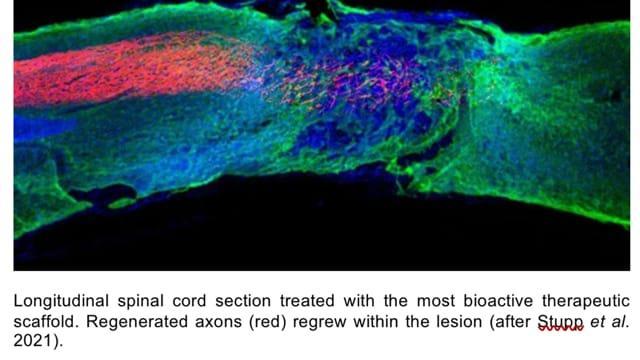 Mice with spinal cord injuries resulting in paralysis to their hind legs, when given a single injection of the therapy regained the ability to walk within four weeks of the treatment compared to those given placebo (dummy) injections. The injections were to the tissues surrounding the spinal cords of the paralysed mice.
Mice with spinal cord injuries resulting in paralysis to their hind legs, when given a single injection of the therapy regained the ability to walk within four weeks of the treatment compared to those given placebo (dummy) injections. The injections were to the tissues surrounding the spinal cords of the paralysed mice.
 The treatment was also of other benefits to severely injured spinal cords by:
The treatment was also of other benefits to severely injured spinal cords by:
Restoring life to damaged extensions of axons (neurones).
Significantly reduces scar tissue formation, which normally forms at the sites of spinal injuries. These scar tissues, studies have found, often create physical barriers to tissue regeneration and repair.
Myelin (a composition of protein and fatty acids), forms an insulating sheath around nerves. It promotes the transmission of electrical impulses along the nerves. When this is damaged, it becomes difficult to transmit impulses. The bioactive therapy was able to cause a reformation of myelin around the cells.
The therapy influences the formation of new blood vessels for delivery of nutrients to cells at the site of the injury.
It also leads to increased survival of motor neurones.
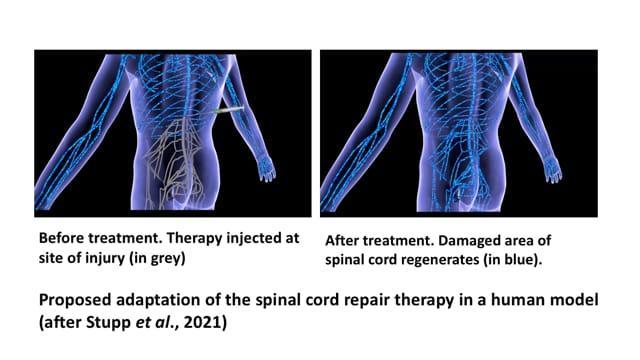
Professor Samuel Stupp who led the study was so optimistic of achieving the same level of success in human models that he announced, “For decades, this has remained a major challenge for scientists because our body’s central nervous system, which includes the brain and spinal cord, does not have any significant capacity to repair itself after injury or after the onset of a degenerative disease. We are going straight to the FDA [United States Food and Drug Administration] to start the process of getting this new therapy approved for use in human patients, who currently have very few treatment options.”
Unlike other therapies designed for spinal cord injury repairs and which leave unpleasant side effects, this treatment is entirely benign and unobtrusive. In fact the artificial nanofibres are biodegradable after the therapy and are used by the body as a source of nutrients for the cells, altogether disappearing from the body without leaving any noticeable side effects.
Already, in tests conducted with human cells, increased bioactivity and cellular signalling were observed, reinforcing the hope that it is possible to translate the success seen in the mouse model not only in the treatment of spinal cord injuries in humans but also in the treatment of other neurodegenerative diseases (spinal muscular atrophy, Huntington’s, Alzheimer’s and Parkinson’s diseases, etc.), and stroke.













Very interesting and well – written article! I learnt something useful!