In an increasingly recurrent battle with coronavirus, effective treatments are urgently needed to reduce the risk of hospitalisation or death. Many vaccines, including monoclonal antibodies and pills, against the COVID-19 condition, have been produced.
But despite all these arrays of weapons deployed against the virus, there are alarming cases of resurgences of the virus in many countries, including Germany, Austria, USA, UK, Denmark, etc. These recent outbreaks are not helped by the emergence and increasing numbers of variants that are resistant to some antibodies. Therefore there is an urgent need for new and improved therapeutic agents to check the spread of the virus and/ or at lease reduce the severe illnesses or death associated with COVID-19 infection.
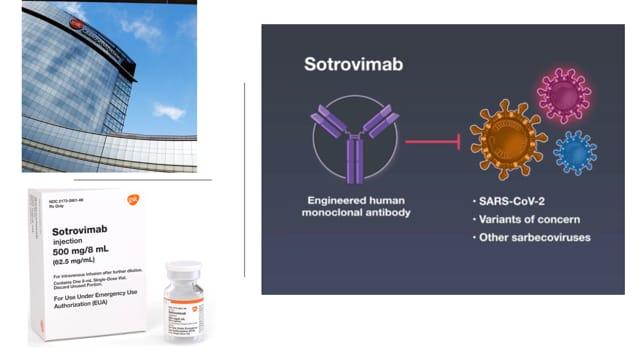
Sotrovimab is an engineered human monoclonal antibody known to be effective in neutralising SARS-CoV-2 (COVID-19) and other SARS-associated Coronavirus. The original version of sotrovimab called S309, was isolated from a patient during the SARS (severe acute respiratory syndrome) outbreak in 2002 – 2004.
Join our WhatsApp ChannelSotrovimab is marketed by GlaxoSmithKline and Vir Biotechnology Inc under the brand name Xevudy. It is specially adapted for use against COVID-19 in high-risk patients immediately after infection. Sotrovimab is also effective against many variants of the virus, including the alpha, beta, gamma, delta, and lambda variants.
READ: Spinal Cord Repair Therapy: Paralysed Mice Walk Again
The therapeutic agent does contain a two-amino acid modification, specially designed engineered to increase its half-life so that its concentration in the body lasts for longer periods of time, enough to engage the virus.
In a recent study published (today) in The New England Journal of Medicine 18 November 2021, by Gupta and coworkers, a double-blind, phase 3, randomised trial was carried out among 868 patients from USA, Canada, Brazil and Spain. In the study, which started on 27 August 2020 and lasted to 4 March 2021, test subjects were selected based on certain criteria: they all were diagnosed with COVID-19 within 5 days before participating in the study, and were at the high risk spectrum i.e. they were 55 years old and above, or were either diabetic, had heart disease, chronic kidney disease, COPD (chronic obstructive pulmonary disease), or moderate to severe asthma.
A dose of 500 mg of sotrovimab was administered in a single 1-hour intravenous infusion to 291 patients, while 292 patients were given a saline placebo (dummy).
The study shows that only 1% of patients given sotrovimab had a disease progression leading to hospitalisation or death, compared to 7% in the placebo group. And 5% of the placebo group were admitted to intensive care unit with one death at day 29 of the test. But no death was recorded in the sotrovimab group.
Safety assessment conducted in 868 patients (430 in sotrovimab group and 438 in placebo group), over a 56-day period, shows serious adverse events occurring in 2% of those given sotrovimab as against 6% of those who were given placebo. But most of these safety issues were not related to sotrovimab but to the COVID-19 condition.
The study found that sotrovimab reduces the risk of hospitalisation or death by 85% compared to those who received placebo. Moreover, non of the patients treated with sotrovimab were admitted to intensive care unit whereas 5 patients given placebo ended up in the ICU. In other words, sotrovimab is effective in preventing severe complications arising from COVID-19 as well as reducing hospitalisation. And most importantly, no safety concerns with regard to the therapeutic agent was observed.
However it must be pointed out that this was a provisional study and did have limitations. For example given that only 1% test subjects were hospitalised in the sotrovimab group, it makes it impossible to know whether hospitalisation was a result of failure of sotrovimab treatment or that of the COVID-19 disease condition.
Also the number of test subjects given sotrovimab during the safety assessment, needs to be expanded in order to pick up any uncommon adverse events. Although the safety of the therapeutic agent the researchers stressed, was not an issue because sotrovimab was derived from an antibody recovered from a SARS-CoV-1 patient who made full recovery.
And finally when one is infected with COVID-19, the resident immune system reacts to the virus. The baseline reaction of the innate immune system is not known. Therefore, its effect on the safety and potency of sotrovimab is not determined.
However the researchers hoped to address some of these issues in their subsequent trials, including a consideration for intramuscular administration.
The study does show that not only is sotrovimab an effective therapeutic agent, but it is a unique neutralising antibody given its mode of activity. Sotrovimab is unlike other monoclonal antibodies, which target the receptor-binding-motif of the virus and block the ACE2 (angiotensin-converting enzyme 2), the receptor used by the virus to gain entry into host cells.
The ACE2 region is very immunogenic and most mutations occur in the region, making it impossible for some receptor-binding monoclonal antibodies to be effective against the emergent variants. But sotrovimab is a monoclonal antibody that neutralises all starbecoviruses (i.e. groups of respiratory viruses), and by targeting conserved regions of the epitope (the site in the virus where an antibody attaches itself) as was done by the researchers, it meant that the functionality or effectiveness of sotrovimab would be retained despite any future evolvement of the virus.
Sotrovimab is particularly good news for COVID-19 outpatients, and because sotrovimab is known to have activity against many variants of the virus, it is therapeutically relevant in the current outbreaks of the disease.
Meanwhile GlaxoSmithKline and Vir Biotechnology Inc are laughing to the bank. They have just signed a contract worth a mouthwatering $1 billion to supply doses of sotrovimab to the U.S. government in the next one month. Other rich countries have also signed contracts with the two companies. In total 750 doses of sotrovimab have been ordered to date.
Dr. Hal Barron, Chief Scientific Officer and President R&D of GSK, was very happy with the deal as he gushed, “Given the large number of patients who continue to become ill with COVID-19 across many regions in the US, there is an ongoing need for access to effective treatments. We are proud to work with the US government to help make sotrovimab available for these patients.”
Dr George Scangos, Chief Executive Officer of Vir Biotechnology Inc, was equally enthusiastic when he said, “Monoclonal antibodies play an essential role in the treatment of certain patients with COVID-19, and we’re grateful that this agreement will allow more healthcare providers and patients who are at high risk for progression to severe COVID-19 to access sotrovimab. Given ongoing evidence, which demonstrates its ability to maintain activity against the tested circulating variants of concern, including Delta, we are confident sotrovimab will continue to be important in the fight against COVID-19.”



















Follow Us