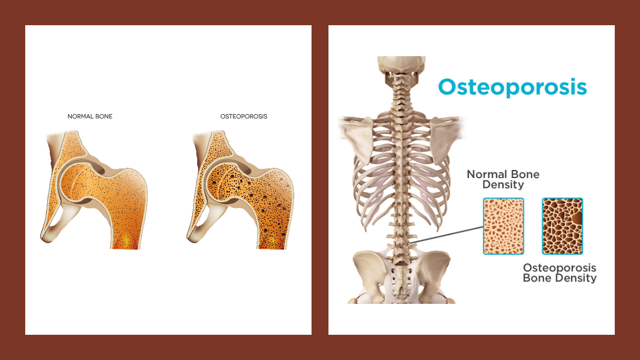
Osteoporosis is a condition in which the bone is brittle and breaks easily due to faster-than-normal loss of bone density. Such a condition is more common in older people, especially in women, and leads to structural abnormalities and fragility of the skeleton.
The skeleton performs a structural function by providing shape, movement, support and protection for the body and its organs, as well as serving as a reservoir for essential minerals. There are many hormones important for bone formation, growth and maintenance, including the growth hormone, oestrogen, testosterone, calcium-regulating hormones (parathyroid, calcitriol from vitamin D, and calcitonin), etc.
Join our WhatsApp ChannelThe bone undergoes two important events: a modelling process that influences the simultaneous formation of a new bone at one site and the removal of old bone from another site within the same bone. This sculpting process allows individual bones to grow and change position. The second event is called the remodelling process. This process occurs throughout life, especially at the later part of life. The process mainly consists of removal and replacement of bone at the same site so that damage to the skeletal structure caused by stress is repaired, and accumulation of old bone that has lost its elasticity is avoided.
The modelling process is particularly pronounced at the early stage of life while the remodelling process occurs particularly at the later stage of life. But both processes continue throughout life so that the majority of the adult skeleton is replaced approximately every 10 years in response to the weakening of bone. By these two processes, the mechanical strength of the bone is preserved irrespective of the loss of bone mass due to ageing.
The sex steroid hormone, oestrogen, is important for bone growth and maintenance. The level of oestrogen falls in female after menopause. This, in some cases results in rapid decrease in bone density. Too much exercise or dieting can cause cessation of periods for up to 6 months and is a contributory factor for osteoporosis. So, also are an early menopause and undergoing hysterectomy before the age of 45.
Alzheimer’s, Parkinson’s, Huntington’s, and other neurodegenerative diseases have been linked to lower bone mass or skeletal abnormalities. The occurrence of these neurodegenerative diseases is commonly seen in patients with osteoporosis. This reinforces the existence of a crosstalk between the bone and brain, otherwise known as the brain-to-bone signalling.
Regulation of bone homeostasis and remodelling are dependent upon the connectivity and bidirectional communication between the nervous system and bone. The brain regulates bone mass whereas osteocalcin (a bone protein hormone derived from bone-forming cells called osteoblasts), mediates many functions in the brain. It binds to neurones in the brainstem, midbrain and hippocampus. Loss of osteocalcin affects the expression of genes involved in neurotransmitter synthesis and could lead to anxiety, depression and memory loss.
READ ALSO: Gut Bacteria Could Checkmate Cardiovascular Diseases By Reducing Cholesterol Levels In Blood
Work carried out by Holly Ingraham and coworkers, which was published in the January 2019 edition of the journal, Nature Communication, showed that when the oestrogen receptor alpha (or ERα) located in the medial basal hypothalamus was surgically removed in mice, it resulted in the formation of strong and chunky bones in female mice only, but not in males. This indicates the presence of an oestrogen-independent signalling pathway for bone formation.
Neurones called Kiss1 found in the hypothalamus play important role in puberty and fertility. Deletion of ERα neurones in both female mice with intact ovaries and those whose ovaries had been removed, resulted in increase in bone mass, thus showing the importance of oestrogen signalling in bone formation. And when ERα in Kiss1 was deleted, a reverse to the formation of strong and chunky bones was observed, identifying Kiss1 as the central figure in the brain-to-bone circuit. The workers, therefore, speculated that the disruption of the female ERα-dependent brain-to-bone pathway would lead to resources for reproduction and energy expenditure being diverted into bone formation and maintenance.
There is a prioritisation of calcium during lactation due to high demand of calcium for milk production. Calcium is then stripped from bone and diverted to milk production. This causes a significant bone loss. The oestrogen hormone normally fills in the gap whenever there is a reduction in bone mass and density by promoting bone formation, but its level drops dramatically during postpartum (ie about 6 to 8 weeks after birth).
Holly Ingraham and coworkers building on their previous research, reported in the July 2024 edition of the journal, Nature, that they have identified a hormone released in the brain that triggers bone formation in response to bone loss experienced during lactation. This brain-derived hormone is called the cellular communication network factor 3 (CCN3) and is secreted from KISS1 neurones.
The workers observed an increased level of the CCN3 hormone in lactating mice, which fell after weaning. And when the level of the hormone is drastically reduced during lactation and accompanied with a low-calcium diet, there was pronounced loss of bone mass after giving birth, and the nursing offspring showed delayed growth and increase in mortality.
READ ALSO: New Research Finds Cure For Spinal Cord Injury
When the workers gave male and non-lactating female mice injections of the hormone over many weeks, there was increase in bone mass of both sexes. Accelerated bone repair was observed when the hormone was introduced into slow-releasing patches placed onto fracture injuries in mice. Also, when the CCN3 hormone was boosted in female mice that have had their ovaries surgically removed in order to reduce oestrogen levels, there was a significant increase in bone mass compared with controls. In effect, in the absence of oestrogen, Kiss1 neurones are activated to release CCN3 hormone. This chemical acts as a bone-boosting hormone by increasing the number of skeletal stem cells.
The exceptional effect of the CCN3 hormone in accelerating fracture repair and stimulating bone formation and homeostasis, could be exploited in the treatment of osteoporosis, especially in lactational osteoporosis. However, answers relating to the relationship between calcium signalling and regulation of the CCN3 hormone’s lactation-dependent release in Kiss1 neurones, will need to be provided, amongst other things.