Infertility, or the inability to conceive, is a fundamental problem that plagues millions of people of both sexes. However, current scientific advancements have led to the development of innovative technologies aimed at addressing the issue.
Reproductive effectiveness in women declines about 10 years before the advent of menopause with average menopausal age recorded at 50 years. There are many contributory factors to the early onset of menopause, including low birthweight, genetics, and environmental factors. Also, diseases such as type 2 diabetes, cancer, and impaired bone health, affect reproductive age.
Join our WhatsApp ChannelREAD ALSO: Anti-Obesity And Diabetes Drugs May Also Enhance Fertility
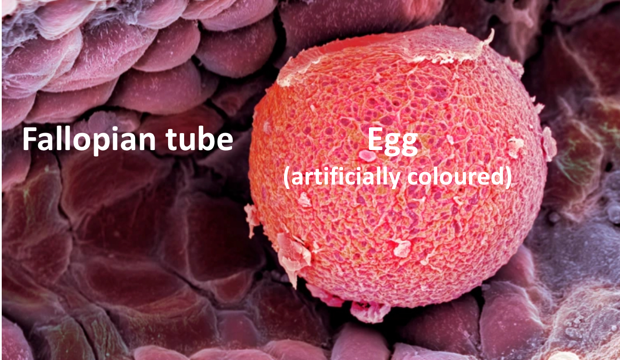
Women have a non-renewable ovarian reserve at birth. This ovarian reserve is established during foetal development and is continuously exhausted throughout reproductive life. Once the reserve is depleted, menopause sets in. The timing of menopause is dependent upon the fidelity of the oocytes (or immature egg cells) in the ovary. The oocytes are subject to quality checks resulting in the body destroying oocytes that contain damaged DNA. Therefore, ability to sustain, detect, repair, and respond to acquired DNA damage is key to healthy oocytes.
READ ALSO: Lactation Hormone May Be Key To Osteoporosis Treatment
Age plays a significant role in ovarian oocyte depletion. The more a woman advances in age, the more the process of oocyte depletion speeds up. Increase in age reduces the effectiveness of the DNA repair mechanisms allowing for accumulation of oocytes containing damaged DNA. The size of the oocyte pool and rate of loss contribute to differences in the onset of menopause seen amongst women.
Heritable genetic disorder and mutations in genes regulating oocyte DNA damage response have major effects on the timing of menopause. It has been reported that mothers who subsist in obesogenic diets (i.e. foods that contribute to weight gain) during pregnancy causes a reduction in ovarian reserve of their female offspring.
In this modern age, there are many women at work with some choosing to delay childbearing in favour of building their career. The typical strategy is to preserve their oocytes and ovarian tissues. But this technique does not always guarantee pregnancy, especially as pregnancy with frozen and then thawed mature oocyte decreases with age of storage. Moreover, the invasive nature of the technique coupled with its dismal success rate of about 6.5% make it very unpopular.
Kari Stefansson and coworkers writing in the August 2024 issue of the journal, Nature Genetics, reported the identification of a variant gene associated with age of menopause. Using a technique called genome-wide association studies (GWAS), they screened 174,329 postmenopausal women from Iceland, Denmark, UK, and Norway. The extracted data showed that women having double copies of a variant of a gene called CCDC201 reached menopause 9 years earlier than other women. This genetic variant is called CCDC201 stop-gain or CCDC201 loss-of-function. The gene variant, which is present in one in ten thousand of women of Northern European descent, causes primary ovarian insufficiency (ie premature menopause) in half of them. Women having double copies of this gene or homozygous for the gene have fewer children and ceased natural fertility 5 years earlier than others. The CCDC201 gene, which was discovered in humans in 2022, is highly expressed in oocytes and is suspected to be important in oocyte survival.
The study found that many non carriers of the trait and heterozygous mothers (i.e. mothers who inherited two different copies of the CCDC201 gene variant from each parent), have children after the age of 30 while only a small proportion of homozygous women (i.e. women who inherited the same version of the gene variant from both parents), have children after the age of 30. Also, non carriers and heterozygous women have more children than homozygous women who in most cases are likely to have only one child or non at all. In other words, homozygous women for the CCDC201 gene variant are predisposed to infertility.
Therefore, identification of carriers of CCDC201 gene variant by genetic screening will aid fertility specialists in devising treatment plans for women predisposed to early menopause.
In the September 2024 issue of the journal, Nature, Anna Murray and coworkers looking for rare genetic variants that might shed more light on ovarian ageing, reported genetic links between ovarian ageing and susceptibility to cancer. In their study, they mined information from a vast collection of biomedical data stored in the UK Biobank, including DNA-sequence data and information about lifestyle and health of participants.
In their study, the workers found 5 new rare protein-coding gene variants associated with ovarian ageing. Women with genotypes of these gene variants experience the onset of menopause more than 5 years earlier than normal, and in some cases more than a year later, depending upon the type of gene variant the woman is carrying. One of the gene variants was found to shorten reproductive lifespan due to delayed puberty timing and premature menopause.
SAMHD1 is a gene that is involved in maintaining genome stability amongst other functions. Depletion of SAMHD1 correlates with an increase in immune cell signalling in ovarian cancer. Murray and coworkers identified variants in the SAMHD1 gene that are associated with cancer risk in both men (prostrate cancer), and women (hormone-sensitive cancers, including breast and ovarian cancers).
The high number of mutations associated with aging is a predominant characteristic of spermatogenesis in men throughout their adult lives. This also applies to women in the sense that an accumulation of DNA mutation in a woman’s eggs triggers early menopause.
More than 8,000 genetic sequences comprising of a trio of father, mother, and child, were analysed in the study. It was discovered that women at higher genetic risk of experiencing early menopause due to mutations in their eggs were more likely to pass the mutations to their offspring. Thus, proving the link between DNA damage and ovarian age. Caution, though, the same finding could not be replicated with data from a different biobank.
DNA repair mechanism is involved in regulation of ovarian ageing. Depletion of the ovarian reserve is a failure to repair oocytes with DNA damage. SAMHD1 deficiency leads to resistance to apoptosis, meaning that delay in the normal age of natural menopause might be due to depletion of ovarian inhibition, which in turn is a result of disrupted apoptosis. Therefore, for homozygous women with any of these damaging variant genes who are undergoing in vitro (i.e. test tube) fertilisation treatment, stimulation of the ovary through limited cell death inhibition, could be an important therapeutic step.
In summary, a slew of these genetic studies may lead to the development of screening tools for damaging genetic mutations and tailored therapeutic interventions in predicting the onset of menopause. These predictive tools should allow for the development of treatments for infertility.