Uncontrollable growth and reproduction of cells in specific areas of the body is known as cancer. The cancerous cells have the tendency to invade and overwhelm healthy cells in nearby tissues and organs, and if left unchecked, the cancer tumour can spread to other areas of the body in a process called metastasis.
There are vaccines available to treat and prevent certain cancers. These vaccines including the human papillomavirus (HPV) vaccine and the hepatitis B vaccine, are aimed at viruses that cause cancer instead of at the cancerous growth; for example, the human papilloma virus causes cervical cancer as well as other sex associated cancers, while hepatitis B infection can lead to liver cancer.
Join our WhatsApp ChannelREAD ALSO: Vaccines for mental health
The first of these cancer vaccines is the hepatitis B vaccine, which is recommended to be administered to babies shortly after birth. Sipukeucel-T is used to treat prostrate cancer, while Bacille Calmette Guerin (BCG) Live intravesical is used to treat early stage of bladder cancer. And another virus-targeting vaccine, Talimigene laherpareovec or T-VEC (marketed under the brand name, Imlygic) is a weakened form of Herpes Simplex Virus Type 1 (i.e. the cold sore virus), and is used to treat difficult-to-operate melanoma (skin cancer).
Cancerous conditions are managed by radiation, surgery or chemotherapy. But the attractive idea is to induce the body to mount an immunity robust enough to attack and destroy the cancerous cells. These types of vaccines (i.e. the therapeutic cancer vaccines), are used in immunotherapy treatments, and are given to people who already have cancer unlike the virus-targeting vaccines, which are administered before one gets the cancer.
The therapeutic cancer vaccines are designed to destroy any remaining cancer cells in the body after the end of treatments with radiation, surgery or chemotherapy. They can also prevent the cancer from resurgence, growing or spreading to other parts of the body.
Cancer cells have signatures called cancer-specific antigens on their surfaces. Healthy cells lack these antigens so that cancer vaccines teach the immune system to recognise the cancer signatures and destroy them.
But some cancer vaccines are tailored to individuals by using the individual’s tumour samples removed during surgery to make the vaccine. An example of this type of vaccine is Sipuleucel-T (sold under the brand name, Provence). It is a cell-based cancer immunotherapy for use in treating metastatic prostate cancer. The autologous cellular immunotherapy process involves harvesting the white blood cells from the patient, modifying the harvested cells in the lab and re-injecting the modified cells back into the patient. The modified cells act as vaccines by teaching the patient’s immune system how to destroy prostrate cancer cells.
The cancer vaccines as aforementioned, unlike other types of vaccines, which prevent infection, target the cancer cells that are already present in the body by initiating and increasing the immunological response of the body immune system against the cancerous cells. But such high expectations of cancer vaccines rarely translate into fruition. This is because cancer cells have low immunogenicity.
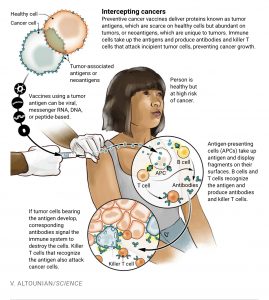
Scientists in the cancer research area are experimenting with various vaccine approaches like the use of cancer-specific (or tumour) antigens; and the use of neoantigens found in tumour cells that induce strong antigenic reactions. Antigen-presenting cells take up antigen and display fragments of the antigen on their surfaces so that the body immune system of B cells and T cells will recognise the antigen and produce antibodies and killer T cells. Many viral, mRNA, DNA, or peptide-based cancer vaccines make use of tumour antigen.
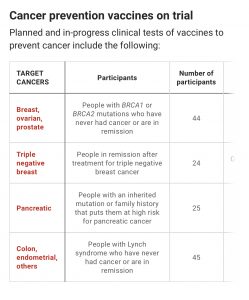
Laboratory and clinical trials of these cancer vaccines are promising, and if successful, will pave the way for developing vaccines to prevent various types of cancers, much as mRNA COVID-19 vaccines have helped in the fight against COVID-19.
But not all cancers are caused by virus, for example, Lynch syndrome (LS), is a rare genetic condition in which the DNA repair gene is faulty due to a mutation. When this happens, the repair DNA is unable to correct errors in dividing cells. This means that errors then pile up as the cells keep dividing resulting in about 70% chances of the person getting cancer of the womb, bowel, stomach, pancreas, ovary, small bowel, or ureter and renal pelvis.
Lynch syndrome increases a person’s risk of many types of cancer including colorectal cancer (cancer of the colon or rectum) and endometrial cancers (cancer of the uterus). The Lunch syndrome in most cases is only discovered during cancer screening. For example, in the US alone, about 1.1 million people carry Lynch syndrome mutations without even knowing.
Microsatellite is a short segment of DNA that is repeated multiple times in succession at a particular area of the genome. These short repeated DNA segments are especially predisposed to DNA mismatch errors. Accumulation of mutations in these microsatellites leads to a condition called microsatellite instability, which is seen in most cancer conditions, especially in colorectal and endometrial cancers.
But a quirk of circumstances meant that microsatellite instability also produces new proteins called neoantigens, which are foreign to the body. These neoantigens can trigger robust immunological reactions against the cancer cells that produce them!
A microsatellite mutation in the gene called the TGFBR2 is seen in about 60 – 80% of people suffering from mismatch repair-deficient colorectal cancer. This means that these shared cancer (or tumour) neoantigens could be a target for developing a cancer vaccine effective for treating millions of people with Lynch syndrome.
Steven Lipkin and coworkers at Weill Cornell Medicine, New York, reported testing a cancer prevention vaccine that targeted Lynch syndrome. The test was done in a mouse model, and the vaccine successfully prevented the growth of colorectal cancers so that vaccinated mice have survival rates greater than unvaccinated ones.
The workers identified 13 shared mutations from 32 colorectal tumours, and using computer algorithms were able to predict that 10 out of the 13 shared mutations would produce neoantigens. Four of the ten neoantigens when injected into mice, triggered a robust immune response. The four positive neoantigens were then combined to create the mouse vaccine. And when this mouse vaccine was administered in conjunction with an adjuvant (a substance that creates stronger immune response), in mice with Lynch syndrome, reduced development of colorectal tumours as well as prolongation of cancer survival in the vaccinated mice were observed as against the unvaccinated mice.
It’s been known that aspirin can decrease the risk of colorectal cancer, although it must be mentioned that in people aged 70 and over, aspirin actually predisposes them to cancer! And naproxen (marketed under the brandname, Naprosyn) used as a common pain reliever and anti-inflammatory medication, has been found to be safe and effective in treating progressive prostate cancer.
In the study, Lipkin and coworkers found that mice treated with the vaccine combined with naproxen lived longer than mice that received only the vaccine or the vaccine plus aspirin. And further, those mice who received the vaccine in combination with naproxen produced more immune cells that recognised the vaccine than did those mice who received only the vaccine or received the vaccine plus aspirin. Thus, a combination of the vaccine, neoantigen, and the painkiller, naproxen, is more effective than administering the vaccine alone or with aspirin.
It is still a long way off before such studies could be replicated in humans, but it is a promising start, and one that gives hope to many that one day cancer will be beaten.


















Follow Us